
PICO, Cochrane and Schema.org
A few weeks ago Cochrane launched their COVID-19 Study Register and I described the data and technical architecture in detail here.
I described how PICO graphs are used to augment the Studies with structured linked data that describe the Population, Intervention, Comparator and Outcome of the trial. Cochrane have now started in earnest annotating PICO graphs onto the Studies, and these are now being surfaced in the Study Register application.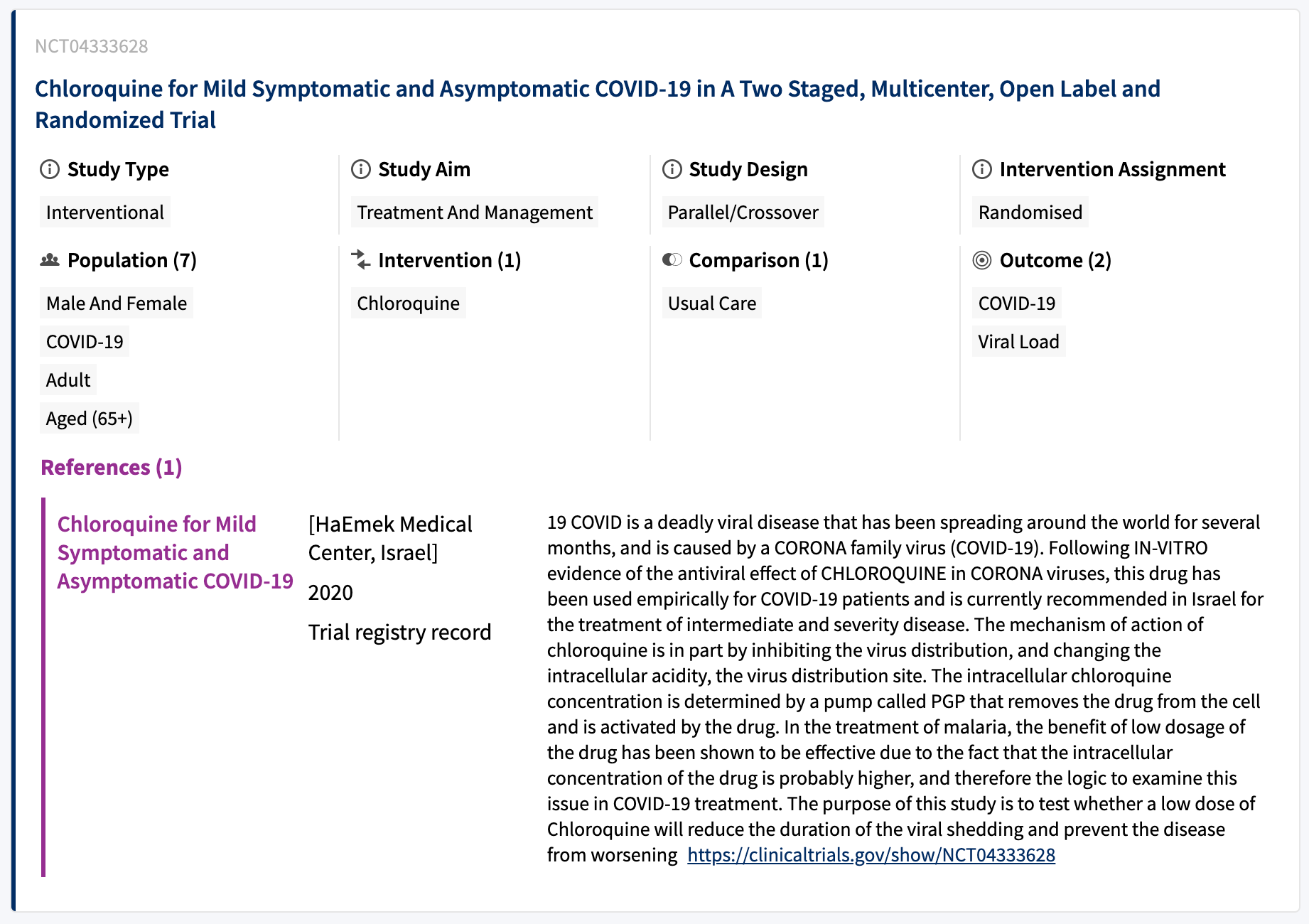
Here, we can see the labels of the PICO concepts rendered in the UI, but in the data itself, these are arranged as a PICO graph structure conforming to the PICO ontology.
We have also added faceted search by PICO concept into the sidebar. This lets users quickly filter down their search results using the Cochrane vocabulary terms.
Once we have a critical mass of PICOs annotated in the register, each vocabulary concept will be searchable in the search bar at the top of the app, extending the previously full-text search into a controlled-vocabulary search. A user can now find studies using a combination of terms chosen from the Cochrane linked data vocabulary, to deliver extremely accurate results. Moreover we can combine a metadata driven search with full-text where needed.
Schema.org
Each Study page rendered in the application is also marked up with schema.org structured data. So while internally we are describing our content and PICO data with the Cochrane bounded context ontologies, at render time, we are transforming our model to the schema.org MedicalEntity model to provide machine readable content for search engines and other content consumers. You can read more about the schema.org medical schema here.
As an example, for this Study https://covid-19.cochrane.org/studies/crs-13266121 when viewing the source of the page, you can see we are marking up the clinical trial as a MedicalStudy in the schema.org ontology, and identifying the healthCondition terms, and the interventions, including the Drug Chloroquine. Each term has associated medical codes mapped to bioinformatics taxonomies including SNOMED-CT, MeSH, RxNorm and the ATC codes for Drugs where available. The resulting schema.org JSON-LD for this study is :
{
"@context": "http://schema.org",
"@graph": [
{
"@type": "MedicalStudy",
"id": "http://data.cochrane.org/studies/crs-13266121",
"name": "NCT04333628",
"identifier": "NCT04333628",
"description": "Chloroquine for Mild Symptomatic and Asymptomatic COVID-19 in A Two Staged, Multicenter, Open Label and Randomized Trial",
"mainEntityOfPage": {
"@type": "WebPage",
"url": "https://covid-19.cochrane.org/studies/crs-13266121",
"headline": "Chloroquine for Mild Symptomatic and Asymptomatic COVID-19 in A Two Staged, Multicenter, Open Label and Randomized Trial",
"description": "Clinical study : Chloroquine for Mild Symptomatic and Asymptomatic COVID-19 in A Two Staged, Multicenter, Open Label and Randomized Trial"
},
"healthCondition": [
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"url": "https://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"name": "COVID-19",
"alternateName": [
"COVID-19",
"Coronavirus disease 2019",
"2019-nCoV acute respiratory disease",
"Wuhan flu",
"HCoV-19",
"SARS-CoV2",
"SARS CoV2 infection"
]
},
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"url": "https://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"name": "COVID-19",
"alternateName": [
"COVID-19",
"Coronavirus disease 2019",
"2019-nCoV acute respiratory disease",
"Wuhan flu",
"HCoV-19",
"SARS-CoV2",
"SARS CoV2 infection"
]
},
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/B5JrjqYxGmF8me",
"url": "https://data.cochrane.org/concepts/B5JrjqYxGmF8me",
"name": "Viral load"
}
],
"studySubject": [
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"url": "https://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"name": "COVID-19",
"alternateName": [
"COVID-19",
"Coronavirus disease 2019",
"2019-nCoV acute respiratory disease",
"Wuhan flu",
"HCoV-19",
"SARS-CoV2",
"SARS CoV2 infection"
]
},
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"url": "https://data.cochrane.org/concepts/NrO30O5ZnYIkjE",
"name": "COVID-19",
"alternateName": [
"COVID-19",
"Coronavirus disease 2019",
"2019-nCoV acute respiratory disease",
"Wuhan flu",
"HCoV-19",
"SARS-CoV2",
"SARS CoV2 infection"
]
},
{
"type": "MedicalCondition",
"id": "http://data.cochrane.org/concepts/B5JrjqYxGmF8me",
"url": "https://data.cochrane.org/concepts/B5JrjqYxGmF8me",
"name": "Viral load"
},
{
"type": "MedicalProcedure",
"id": "http://data.cochrane.org/concepts/r4hp5z141f1t",
"url": "https://data.cochrane.org/concepts/r4hp5z141f1t",
"name": "Usual Care",
"alternateName": [
"Routine Prenatal Care",
"Routine Care",
"Standard Care",
"Providing care according to standard",
"SOC"
],
"code": [
{
"codingSystem": "SNOMED-CT",
"codeValue": "372921003"
}
]
},
{
"type": "Drug",
"id": "http://data.cochrane.org/concepts/r4hp13k8jxft",
"url": "https://data.cochrane.org/concepts/r4hp13k8jxft",
"name": "Chloroquine",
"alternateName": [
"Chloroquine",
"Product containing chloroquine"
],
"code": [
{
"codingSystem": "MeSH",
"codeValue": "D002738"
},
{
"codingSystem": "SNOMED-CT",
"codeValue": "14728000"
},
{
"codingSystem": "RxNorm",
"codeValue": "2393"
},
{
"codingSystem": "ATC",
"codeValue": "P01BA01"
}
]
},
{
"type": "MedicalEntity",
"id": "http://data.cochrane.org/concepts/kn3ptfq7c6lz",
"url": "https://data.cochrane.org/concepts/kn3ptfq7c6lz",
"name": "Pharmacological Interventions"
},
{
"type": "MedicalEntity",
"id": "http://data.cochrane.org/concepts/kn3ptfq7c6m7",
"url": "https://data.cochrane.org/concepts/kn3ptfq7c6m7",
"name": "Other"
},
{
"type": "MedicalEntity",
"id": "http://data.cochrane.org/concepts/q25g9q497cwj",
"url": "https://data.cochrane.org/concepts/q25g9q497cwj",
"name": "Physiological or clinical",
"code": [
]
},
{
"type": "MedicalEntity",
"id": "http://data.cochrane.org/concepts/q25g9q497cwj",
"url": "https://data.cochrane.org/concepts/q25g9q497cwj",
"name": "Physiological or clinical",
"code": [
]
}
],
"subjectOf": [
{
"@type": "MedicalScholarlyArticle",
"datePublished": "2020-01-01",
"url": "https://clinicaltrials.gov/show/NCT04333628",
"headline": "Chloroquine for Mild Symptomatic and Asymptomatic COVID-19",
"abstract": "19 COVID is a deadly viral disease that has been spreading around the world for several months, and is caused by a CORONA family virus (COVID-19). Following IN-VITRO evidence of the antiviral effect of CHLOROQUINE in CORONA viruses, this drug has been used empirically for COVID-19 patients and is currently recommended in Israel for the treatment of intermediate and severity disease. The mechanism of action of chloroquine is in part by inhibiting the virus distribution, and changing the intracellular acidity, the virus distribution site. The intracellular chloroquine concentration is determined by a pump called PGP that removes the drug from the cell and is activated by the drug. In the treatment of malaria, the benefit of low dosage of the drug has been shown to be effective due to the fact that the intracellular concentration of the drug is probably higher, and therefore the logic to examine this issue in COVID-19 treatment. The purpose of this study is to test whether a low dose of Chloroquine will reduce the duration of the viral shedding and prevent the disease from worsening",
"author": [
{
"type": "Person",
"name": "HaEmek Medical Center"
}
]
}
]
}
]
}PICO and Schema.org
The PICO model and process (https://en.wikipedia.org/wiki/PICO_process) is now widely adopted for framing research questions in evidence based healthcare with the BMJ citing this model as best practice .
PICO data is applied by clinicaltrials.gov in a semi-structured tabular format on most of their published trials and PubMed also has a PICO search tool for querying MEDLINE, the database from the US National Library of Medicine.
Supporting PICO would be a welcome addition to the schema.org model, allowing any publisher of medical literature to mark up their content in a standard way with machine readable structured PICO data as Cochrane and others are now. I can imagine a future where Google indexes the schema.org PICO markup and provides the ultimate PICO based search tool across all the medical literature citations at their disposal.
At Data Language, as co-authors of the PICO Ontology, we would be more than happy to help define some simple extensions to the schema.org MedicalEntity model to make this happen.

